The PICASO Newsletter #4 is now available! In this number, we take a closer look at how the ethical and legal aspects of the project have been managed.
The use of monitoring techniques to collect patient data: A legal perspective
PICASO has had the advantage of having a legal expert from VUB as a project partner. In addition to sitting on the Ethical Board, VUB has advised the consortium on the applicable regulatory frameworks and legal requirements, both in the context of ‘PICASO as a research project’ and in the context of ‘PICASO as an exploitable system’. In particular, the consortium was advised on legal issues that related to surveillance issues and to the use of patient data in a manner that was foreseen by the PICASO project.
Moreover, the legal requirements in relation to data privacy and data protection as the General Data Protection Regulation (GDPR) came into force on 25 May 2018 (i.e. halfway into the project) were carefully analysed to ensure that the project complied. Finally, the potential issues that might arise as a result of Medical Device Regulation were also carefully considered and discussed within the project. A detailed analysis of the legal issues in the context of PICASO are provided in D3.5 Privacy Compliance Laws Associated with Surveillance.
To fully understand the legal framework and requirements related to PICASO, it was first of all necessary to conceptualise PICASO in two forms: i) ‘PICASO as an exploitable product’ which refers to a platform architecture that potentially will be deployed after the PICASO project has been finished and ii) ‘PICASO as an exploitable system’ which refers to the described trials in two different legal jurisdictions.
‘PICASO as an exploitable product’
The goal of the PICASO is to demonstrate that the federation of patient data is possible in a way that respects ethical and legal concerns surrounding privacy. The diagram bellow shows one possible way to view the architecture of the project.
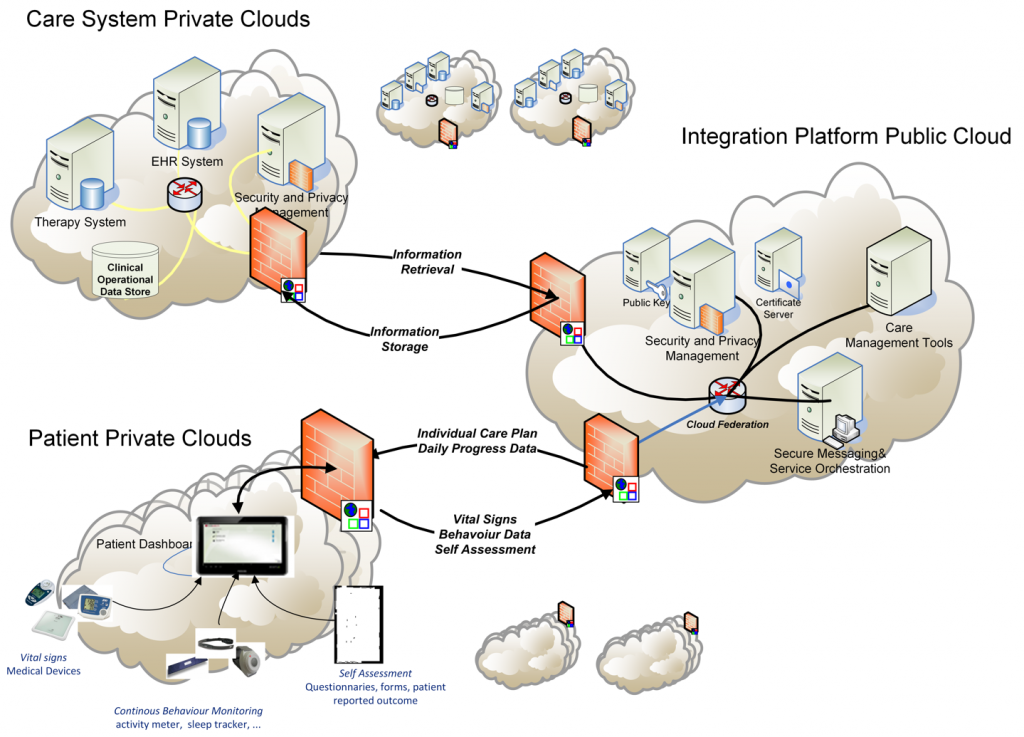
This view of the architecture demonstrates some key features of a potential PICASO system that may be used. Some of the most important elements that are important from a legal perspective are: i) Patient data is not stored centrally in a cloud, ii) A number of physicians or carers may have access to a patient’s data, and iii) Individual patients will have control over which physicians can see their data and what they can see, and iiii) (iv) This can include data that comes from mobile monitoring devices.
‘PICASO as an exploitable system’
The aim of the PICASO project is to demonstrate the possibility of such an infrastructure (as described above) on a small scale in the context of two controlled trials in Germany and Italy. These trials will involve the communication of data between specialists of two different categories in other to demonstrate the principles discussed above. In both instances all data will be transferred between the local hospital site and other care providers through the use of a local cloud service provider. This provider will be based in the same jurisdiction as the trial is taking place. All processing will be in compliance with local data protection law and ethical requirements. This involved getting clearance from local ethics committees and producing consent forms for trial participants in line with both the demands of the local ethic committees and the demands made by the PICASO ethical board.
To find out more see D3.5 Privacy Compliance Laws Associated with Surveillance.
Surveillance, Stigma and Dignity
The ethics of surveillance raises a number of issues but in PICASO we have focused on stigmatisation and human dignity. Both issues are extremely important in the context of healthcare in general and with specific focus on the patient, notably a chronic patient, in particular. Surveillance, monitoring, stigmatisation and human dignity cannot be separated from a discussion on privacy and protection of personal data, especially as the digitalisation of societies, and of healthcare, increases and spreads.
Deliverable D3.4 Ethical Analysis of Monitoring and Privacy Impact Assessment carried out an ethical analysis of these issues. The analysis was placed in context of the PICASO home-monitoring solutions used by the patients in the two trials. Patients tested and used the home-monitoring solution:
1) to measure and monitor blood pressure, weight and activity (steps per day) using the provided devices and the PICASO App for transferring data to the clinic
2) to monitor well-being and medication adherence with the use of very short questionnaires that were answered directly in the PICASO Patient Dashboard.
The analysis will help the reader understand why it is important to consider the ethical aspects of using surveillance and monitoring technologies in a healthcare context and how the PICASO project has approached these issues. The concept of (human) dignity was also be analysed as it is central to our understanding of privacy and data protection and the threats to these in a digital age. There is a clear link between the analysis and the Ethical Guidelines and Principles in the project.
The deliverable D3.4 Ethical Analysis of Monitoring and Privacy Impact Assessment will be available for download in the “Knowledge Centre” menu after the project’s final review scheduled for August 2019.
The PICASO Ethical Guidelines
The PICASO Ethical Guidelines were based upon the project’s Ethical Principles. The guidelines were implemented in the project by means of the development of a check list, The PICASO Ethical Check List. The Check List is a tool for the project to check if the Ethical Principles were implemented and that applicable regulatory requirements were fulfilled.
The Check List was formatted as a series of questions aimed to ensure that the trials were conducted in an ethically sound manner with respect to the involvement of patients and their personal data. The Check List was used to assess compliance with the Ethical Guidelines as well as relevant data protection and privacy regulations. In fact, annual compliance and monitoring reports (internal reports) were completed which were largely based upon the results of completed Check Lists. Both trials have shown compliance with the Ethical Guidelines and the PICASO Ethical Board has also approved the annual compliance reports indicating that the PICASO project and in particular the two project trials have been conducted in an ethically sound manner.
The PICASO Ethical Guidelines and the Ethical Check List are available in D3.3 The PICASO Ethical Guidelines.
The PICASO Ethical Board
The PICASO Ethical Board was established as an advisory entity soon after the project started. The PICASO Ethical Board consists of the project’s Ethical Manager (Chair), a legal expert, the two trial owners (internal members) and three external expert members.
The PICASO Ethical Board had its first meeting on 25 May 2016 where the ethical issues relevant to the project and the two trials were discussed, the PICASO Ethical Guidelines and Principles were defined, and the board’s Terms of Reference were agreed upon.
Three annual Ethical Board meetings have been held during the project’s lifetime. The board meetings have been used to discuss the ethical concerns/challenges either foreseen or encountered and advise on the best resolution. The meetings were actually held prior to active involvement of patients in the trials and thus represented a good opportunity for ensuring that any ethical and/legal issues were resolved before the trials started.
In addition, one online meeting was held in February 2019; the main purpose here was to give a final update on the project’s trials and their results as the trials were in their last phase.
The external board members have been very engaged in the project and their comments, observations and suggestions have been helpful and are truly valued by the project. See D3.3 PICASO Ethical Guidelines for more information about the Ethical Board.
The PICASO Ethical Principles
One of the first activities of the PICASO project was to establish a set of ethical principles and guidelines for the project. This was highly prioritised by the project because the two project trials would involve real patients who would test and evaluate the PICASO solutions.
First of all, an initial analysis of the main ethical issues that were relevant for the project and in particular with respect to the involvement of patients in the trials was carried out. The analysis identified seven ethical issues that would need to be carefully considered and monitored during the project: Informed Consent; Autonomy; Dignity; Stigmatisation; Inclusion; Privacy and Data Protection; Clinical Incidental Findings. The list was by no means exclusive nor did it imply that other ethical issues would not be considered nor dealt with in the project. The issues should also not be seen as completely separated; they may be interlinked in various ways, as well as implicating other matters of an ethical nature. As these issues were considered the most relevant ethical issues, they are closely linked to the Ethical Principles.
Secondly, the Belmont Report was consulted as it represents a valuable reference for defining ethical principles for research involving human subjects. The Belmont Report was created in 1978 by the National Commission for the Protection of Human Subjects of Biomedical and Behavioural Research but it continues to be relevant.
As a result, four Ethical Principle were defined and implemented in the project:
- Respect for Persons
- Beneficence
- Justice
- Respect of Confidentiality and Privacy
In order to ensure that the consortium had a common understanding of the practical implications of the Ethical Principles, concrete examples of how the principles should be applied in the project were also described. The principles and their application were discussed with the PICASO Ethical Board prior to being finally agreed to by the consortium.
For more information on the project Ethical Principles please see deliverable D3.3 The PICASO Ethical Guidelines.
Presentation of PICASO at DGIM 2019
The abstract ‘PICASO – Die Plattform für eine verbesserte persönliche, koordinierte Betreuung chronisch Kranker’ will be presented at the DGIM by Heinrich-Heine-University Düsseldorf, University Clinic Düsseldorf. The poster presentation is part of the theme, Digital Medicine, which will be presented on Monday 6th May.
The DGIM Congress takes place on 4-7 May 2019 at the RheinMain Congress Center in Wiesbaden, Germany.
PICASO at the XIV Congresso Nazionale AIMN 2019
Agostino Chiaravalloti from University of Rome “Tor Vergata” Hospital will present PICASO at the AIMN congress in Rimini, Italy, on 12 April 2019. This year, participants at the AIMN congress will earn Continuing Medical Education (CME) credits and is therefore very exciting to be able to present PICASO in this context. Moreover, audiences will be presented with a questionnaire on PICASO, allowing us to gather a unique insight into how PICASO is received by a wide professional audience.
Project Videos
Two different project videos are currently being produced by a professional film production company, MasterMedia. The film crew was recently in Rome to film interviews with professionals and patients on their experiences with using PICASO for the sharing of care plans and patient data. The video will demonstrate how PICASO helps to overcome the fragmentation of today’s health care systems by providing a data platform that ensures that all relevant information will reach patients and professional caregivers across care sectors on time.
The other project video will be an animation video that will demonstrate the PICASO home-monitoring solution from the patient perspective and how it supports patient adherence to the care plan. The video will demonstrate the PICASO tools that ensures an active inclusion of the patient as well as an easy way to communicate with other healthcare professionals based on safe data transfer.
PICASO innovation in the final for the Innovation Radar Prize 2018
Project partner CNet has been selected as one of 50 finalists for the European Commission‘s Innovation Radar Prize 2018 with the PICASO innovation: Federated Cloud Architecture designed for ehealth: Care Management as a Service.
The Innovation Radar Prize is a prestigious prize that puts a spotlight on high quality innovative excellence, emerging from the Horizon 2020 programme. Finalists are grouped into five categories, with 10 finalists in each category, and CNet has been selected for the “Tech for Society” category, recognising technologies impacting society and citizens.
The innovation
Care Management as a Service is a cloud solution that enables care organisations to use cloud technology and experience benefits of scalability and software resource sharing without violating regulations regarding clinical data. The innovation is based on a software-to-data cloud approach. All software for care management and decision support is hosted in one public cloud while all clinical data always resides inside the care organisations. All access and use of clinical data are done in a secure and authorised way, without violating GDPR.
“This is an innovation that healthcare has been looking for since cloud technology was introduced. We are very proud to have been selected and it is an important recognition of the innovative work being carried out in the PICASO project”, says Peter Rosengren, technical coordinator of PICASO and CEO of CNet.
Vote for your favourite
Thousands of EU-funded innovations from across Europe were scanned in the process, making it quite an achievement to make the final 50. But it is not over yet. The public has to vote for the favourite innovators, who will then pitch their innovation in front of a jury before a winner can be announced.
Vote for CNet’s innovation HERE